News & Opinion
Canada Becomes 3rd Country To Approve PrEP
Greg Broom
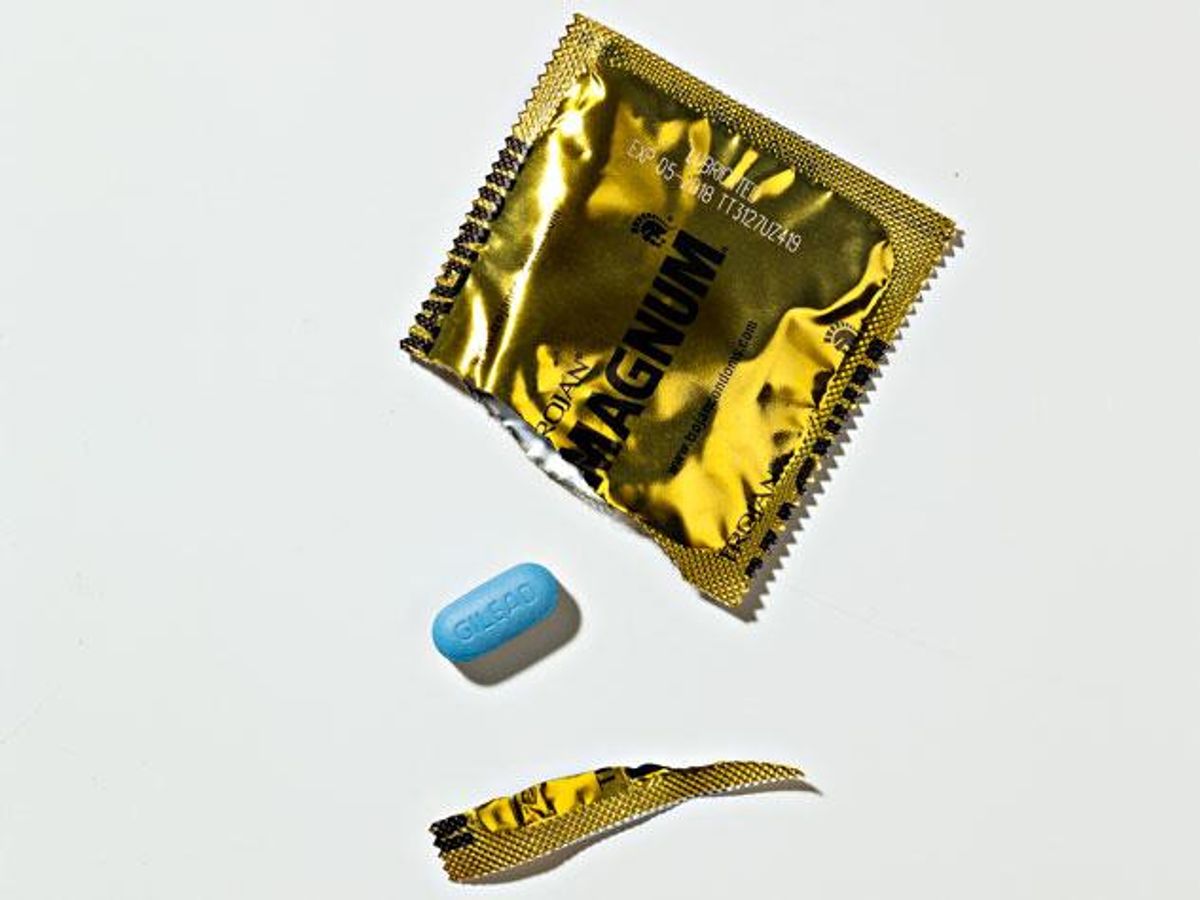
Cananda becomes the third nation in the world to approve PrEP as a treatment plan against HIV.
March 02 2016 4:20 PM EST
October 16 2016 10:09 PM EST
By continuing to use our site, you agree to our Private Policy and Terms of Use.

Cananda becomes the third nation in the world to approve PrEP as a treatment plan against HIV.
Canda's department of health, Health Canada, announced today that Truvada (a.k.a PrEP) has officially been approved as a means to prevent patients from acquiring HIV.
"The drug is intended for use by high-risk individuals --such as those whose sexual partner is HIV positive-- in combination with safer sex practises, including condom use," a Health Canada spokesperson toldBuzzFeed Canada.
"Today's approval of Truvada for PrEP in Canada represents a meaningful advance in Gilead's efforts to address HIV across the entire spectrum, including prevention, treatment, and testing and linkage to care," Norbert W. Bischofberger, Gilead's chief scientific officer, said in a statement.
"We are pleased to offer this important HIV prevention tool to at-risk populations in Canada and we remain engaged with regulatory agencies in other countries around the world that express interest in Truvada for PrEP."
The approval of PreP could lead to lower prices for the drug in Canada, as well as a potential increase in prescriptions.
Meanwhile, other countries face extreme pressure to officially approve PrEP. In the UK, PrEP can only be prescribed "following unsafe sex." As a result, gay men have been known to "clinic hop" in the hopes of discovering a doctor who will prescribe the drug prior, not after, unsafe sex.
PrEP is currently only officially approved in the U.S., Canada, and France.
Cooper Koch and twin bro spark controversy with eye-popping 'White Lotus' parody